When a formulation passes every calculated compatibility check but still blooms after six months in the warehouse, something is wrong with the assessment method. This scenario plays out more often than formulators expect, particularly as the industry shifts toward non-phthalate alternatives.
Compatibility between plasticizer and polymer determines whether your PVC product maintains flexibility, appearance, and performance over its service life. Solubility parameter calculations provide a useful starting point for screening candidates, but they capture only part of the picture. Calculations based on Hansen Solubility Parameters (HSP) achieve roughly 57-59% accuracy for polar polymers like PVC. That leaves a substantial margin for production surprises.
What Determines Plasticizer-PVC Compatibility
Compatibility fundamentally depends on molecular similarity between the plasticizer and PVC. The closer their solubility parameters match, the more readily they mix without phase separation.
PVC has a calculated Hildebrand solubility parameter of approximately 19.7 (MJ/m3)^0.5. A plasticizer functions as a good solvent for PVC when its solubility parameter falls within roughly +/-2 (MJ/m3)^0.5 of this value. Traditional phthalates like DEHP sit comfortably in this range at 17.9-19.2 MPa^0.5.
The Relative Energy Difference (RED) offers a more refined compatibility prediction. When RED falls below 0.4, high compatibility is likely. Above 0.7, poor compatibility becomes probable. Values between 0.4 and 0.7 require testing to confirm.
Molecular weight creates a trade-off. Lower molecular weight plasticizers integrate more efficiently with PVC chains, improving processing and initial flexibility. Higher molecular weight plasticizers resist migration better but require higher loading levels to achieve equivalent softening. Low molecular weight plasticizers under 300 g/mol can migrate 10 times faster than those above 500 g/mol.
Polar interactions also matter for PVC. The chlorine atoms along the PVC backbone create polar sites that interact with polar groups on plasticizer molecules. These specific interactions explain why structurally similar plasticizers can perform differently in PVC formulations.
Solubility parameters provide the starting point for compatibility assessment, not the final answer.
Why Solubility Parameter Calculations Fall Short
Calculations based on solubility parameters predict compatibility for polar polymers like PVC with only 57-59% accuracy. For nonpolar polymers, accuracy reaches 72-77%. The difference matters when your production depends on reliable predictions.
The fundamental limitation is that solubility parameter methods account primarily for enthalpic contributions to mixing. They do not quantify entropic effects or capture specific molecular interactions. As one computational chemistry review notes, this approach “does not provide any quantitative information regarding drug-polymer miscibility as well as the physical state” and “can give misleading results.”
Different calculation methods compound the problem. Group contribution approaches, which build up solubility parameters from molecular fragments, produce different values for the same molecule depending on which method is used. A plasticizer might appear compatible using one calculation approach and marginal using another.
The gray zone presents practical difficulties. When the solubility parameter difference falls between 5.0 and 10.0 MPa^0.5, predictions become unreliable. Many formulations land in this uncertain range where only testing can provide answers.
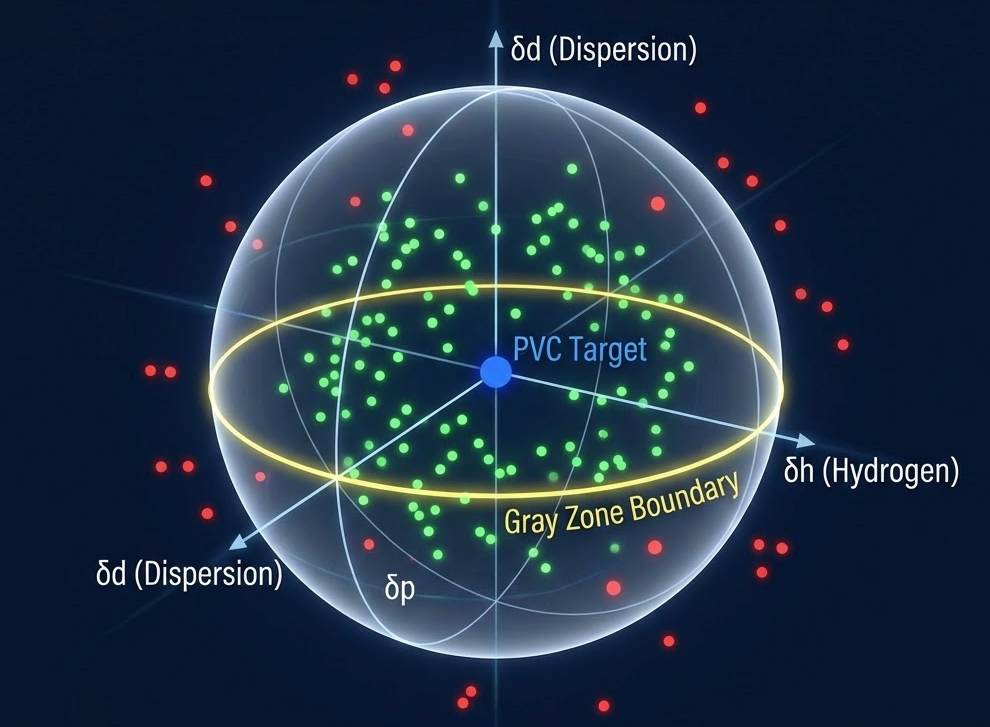
The Hansen model improves on the Hildebrand approach by separating solubility into dispersion, polar, and hydrogen bonding components. This three-dimensional approach achieves 69% average accuracy versus 60% for Hildebrand. Nine percentage points better than a simpler model, but still leaving substantial uncertainty.
A 57-59% hit rate beats random guessing, but production decisions require higher confidence. Calculations serve as efficient screening tools that narrow the field of candidates. Final compatibility confirmation requires physical testing.
Non-Phthalate Plasticizers: The Validation Challenge
Non-phthalate types of plasticizers lack the decades of empirical data that traditional phthalates accumulated. Every formulation using DOTP, DINCH, or bio-based alternatives should be treated as unproven until tested, regardless of favorable calculations.
ExxonMobil’s experience with DOTP illustrates the gap between calculation and reality. The company patented DOTP (also called DEHT) as early as 1953. Despite structural similarity to successful phthalates, compatibility testing revealed problems. “Because of its poor compatibility with PVC, the company decided NOT to commercialize DOTP (DEHT) and shift its focus to other, more reliable solutions,” according to ExxonMobil’s own documentation. They developed DIDP and DINP instead. The same plasticizer rejected in 1953 now sees widespread use as a phthalate-free alternative, often with loading adjustments and formulation changes that address its compatibility limitations.
Bio-based plasticizers present an even sharper contrast between calculated and actual compatibility. Epoxidized soybean oil (ESO) shows the most favorable interaction energy with PVC in molecular dynamics simulations, with delta-E values of -1721 kcal/mol compared to -1486 kcal/mol for DOP. By calculation, ESO should be an excellent plasticizer. In practice, ESO exudes when levels exceed 5-7% of the formulation. Currently, ESO can only replace about 30% of DOP in most PVC formulations, regardless of what simulation predicts.

Blend solutions discovered through testing demonstrate what calculations cannot predict. Researchers found that neither DEHT nor di-n-butyl terephthalate (DnBT) alone matched DEHP compatibility in PVC. However, a 70:30 weight percent DnBT-DEHT blend performed on par with the DEHP control. DnBT acted as an intermediary between PVC and DEHT, improving overall blend compatibility. No calculation method would have predicted this synergy.
These cases share a common thread: testing revealed outcomes that theory missed. For non-phthalate formulations, build your empirical database through systematic testing rather than relying on calculated predictions.
Testing Methods for Compatibility Assessment
Multiple test methods exist because no single test captures all aspects of compatibility. Different tests measure different phenomena, and they show limited correlation to each other. A formulation may pass one test and fail another, which actually provides useful diagnostic information.
The ASTM D3291 loop bend spew test provides a practical screening method. A plasticized PVC strip is bent 180 degrees at room temperature (23+/-2C, 50+/-10% RH) and examined at 4 hours, 24 hours, and 7 days. A cigarette paper wipe detects any surface exudation. Results rate from 0 (no spew) to 2 (significant exudation). This test stresses the material mechanically while revealing migration tendency under ambient conditions.
ASTM D2383 tests compatibility under humid conditions through accelerated aging. Humidity affects migration rate substantially, with high humidity increasing migration up to 50%. This test reveals how formulations will behave in humid storage or service environments.
ISO 177 measures migration tendency by contacting plasticized PVC with absorbent materials under heating. This accelerated test compresses months of ambient plasticizer migration into days.
Simple oven screening at 70-90C for 24-48 hours works as an initial filter. Visual inspection for oiliness, haze, or surface tackiness indicates potential compatibility problems. This low-cost approach catches obvious failures before committing to more rigorous testing.
Temperature accelerates migration exponentially. Migration rate roughly doubles for every 10C temperature increase. Accelerated tests at elevated temperature predict long-term ambient behavior, though extrapolation requires understanding of activation energies for specific plasticizer-PVC systems.
Use at least two different test methods when evaluating a new formulation. If both pass, confidence in compatibility is reasonable. If results disagree, the formulation likely sits in a borderline compatibility range requiring further investigation or reformulation.
Conclusion
Solubility parameter calculations efficiently screen plasticizer candidates and eliminate obvious mismatches. For polar polymers like PVC, expect roughly 60% accuracy from calculated predictions. That accuracy suffices for narrowing options but not for production commitments.
Standardized testing with ASTM and ISO methods confirms what calculations can only estimate. The investment in testing pays dividends through avoided field failures and customer complaints. For any formulation using non-phthalate plasticizers, systematic validation testing is not optional.
Start with HSP calculations to identify promising candidates. Validate compatibility through physical testing under conditions that simulate your application. Build your own empirical database for newer plasticizer chemistries where published data remains limited. The path from calculated compatibility to production confidence runs through the testing lab.