US hospitals use 2.5 million IV bags daily. The flexible PVC tubing delivering those fluids can contain up to 80% plasticizer by weight. For decades, that plasticizer was DEHP (di-2-ethylhexyl phthalate). The regulatory landscape is now shifting toward stricter requirements, and procurement managers navigating medical-grade plasticizer selection face a fragmented maze of FDA guidance, EU MDR mandates, and testing standards.
This guide covers what qualifies a plasticizer as medical grade, how US and EU requirements compare, what testing standards actually require, and which plasticizer fits which application.
What Makes a Plasticizer Medical Grade?
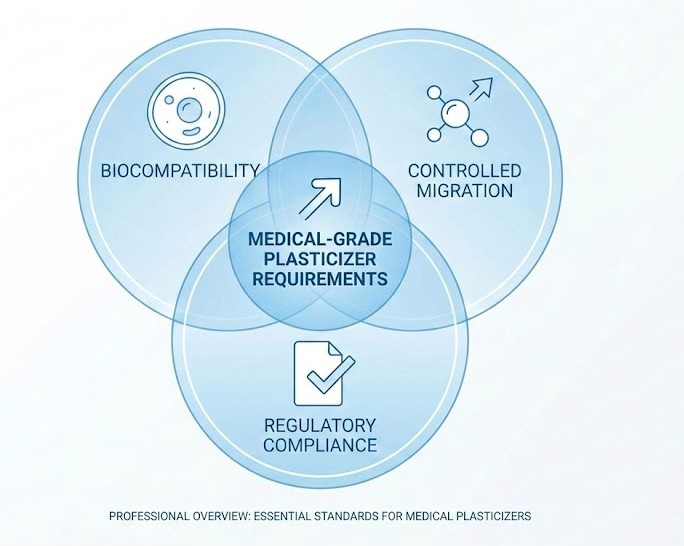
Medical-grade plasticizers must meet three requirements that industrial plasticizers often do not: biocompatibility, controlled migration, and regulatory compliance documentation.
The EU Medical Device Regulation (MDR 2017/745) sets the clearest threshold. Substances classified as CMR 1A or 1B (carcinogenic, mutagenic, or reproductive toxic) cannot exceed 0.1% by mass in devices contacting patients. DEHP falls into this category. For devices requiring higher concentrations, manufacturers must provide justification based on clinical benefit versus risk.
The FDA takes a risk-based approach rather than setting hard thresholds. Two factors determine risk: aggregate patient exposure and sensitivity of the exposed population. FDA guidance establishes Tolerable Intake (TI) values for DEHP at 0.60 mg/kg/day for parenteral exposure and 0.04 mg/kg/day for oral exposure. Devices must demonstrate patient exposure stays below these limits.
The practical difference: EU MDR restricts the material composition, while FDA focuses on patient exposure. A device might comply with one framework but not the other. Compared to traditional phthalates, non-phthalate plasticizers simplify compliance by avoiding CMR classification entirely.
Regulatory Frameworks: FDA vs EU MDR
FDA Approach
FDA does not ban DEHP in medical devices. Instead, guidance recommends labeling devices containing DEHP when they contact patients in high-risk categories: male neonates, pregnant women, and perinatal females. The agency’s 2002 guidance remains the primary reference, though California’s Toxic-Free Medical Devices Act (effective 2030) will impose stricter state-level requirements.
FDA relies on device manufacturers to evaluate biocompatibility under 21 CFR Part 820 quality system requirements. The agency endorses ISO 10993-1 as the framework for biological evaluation. Manufacturers bear responsibility for demonstrating their specific device meets safety thresholds.
EU MDR Requirements
EU MDR 2017/745 takes a precautionary approach. Article 10(4) requires that CMR 1A/1B substances above 0.1% concentration receive justification in technical documentation. The SCHEER (Scientific Committee on Health, Environmental and Emerging Risks) updated guidelines in June 2024 provide current risk assessment methodology.
The July 2030 deadline requires full MDR compliance for all medical devices. Devices grandfathered under previous directives must transition or exit the market.
Where They Converge
Both frameworks are converging toward similar outcomes through different mechanisms. California 2030 and EU MDR 2030 create parallel phase-out pressures. The market trend across regions is clear: DEHP-free formulations are becoming the default specification for new device development.
Compliance requirements now include documentation that would satisfy both frameworks. Specifying to the stricter EU 0.1% threshold typically satisfies FDA requirements as well, simplifying global supply chains.
Testing Standards Explained
ISO 10993 Series
ISO 10993 is the international standard for biological evaluation of medical devices. Part 1 provides the framework; subsequent parts address specific tests. For plasticizers, three parts matter most:
ISO 10993-5 covers cytotoxicity testing. Extracts from materials contact cell cultures; cell death indicates toxicity. This screens for acute cellular harm.
ISO 10993-17 establishes allowable limits for leachable substances. It defines how to calculate Tolerable Intake (TI) or Tolerable Exposure (TE) values based on toxicological data.
ISO 10993-18 addresses chemical characterization. As testing laboratories note, chemical characterization is the first step in biological evaluation. This standard was added to EU MDR harmonized standards in March 2024, making it effectively mandatory for EU market access.
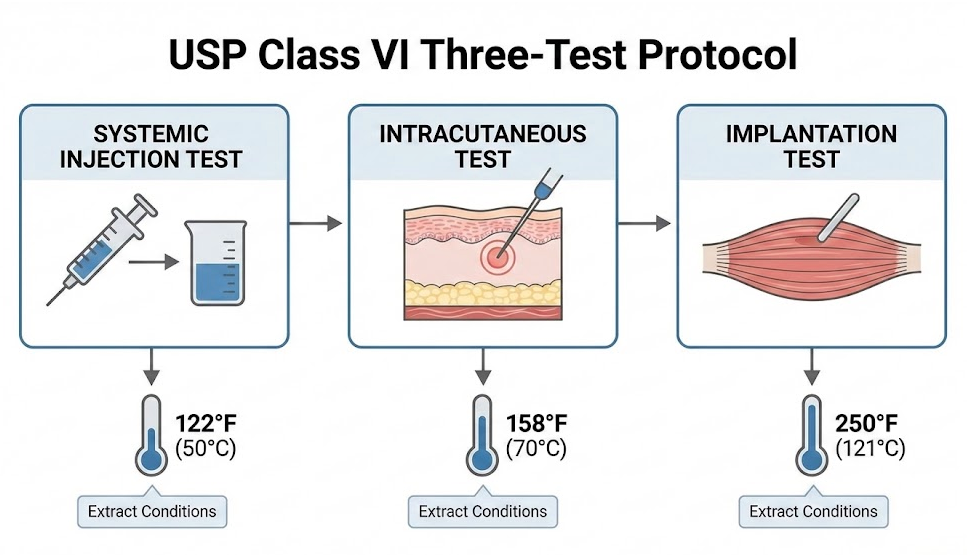
USP Class VI
USP Class VI is the most stringent United States Pharmacopeia classification for plastics. The protocol requires three tests:
- Systemic injection test: Material extracts injected into animals, monitored for 72 hours for systemic reactions
- Intracutaneous test: Checks for local skin reactions at injection sites
- Implantation test: Materials implanted in tissue, response observed for 120+ hours
Extracts are processed at three temperature/time conditions (122F/72h, 158F/24h, 250F/1h) to simulate various use scenarios.
USP updates take effect in stages: USP <665> on May 1, 2026, with USP <88> and <87> updates following on December 1, 2026. These changes align USP more closely with ISO 10993, reducing duplicate testing requirements.
Extractables vs Leachables
Extractables testing uses exaggerated laboratory conditions to identify everything that could migrate from a material. Leachables testing uses conditions simulating actual use to identify what does migrate.
A plasticizer might show high extractables under aggressive solvents but minimal leachables under realistic blood or saline contact. Both data points matter: extractables for safety assessment, leachables for exposure calculations.
Selecting Plasticizers by Application
No single plasticizer works best for all medical applications. The optimal choice depends on device type, patient contact duration, and regulatory requirements.
Migration Rate Comparison
Migration rates vary widely between plasticizer types. Using DEHP as the baseline:
| Plasticizer | Migration Rate vs DEHP | Best For |
|---|---|---|
| DINCH | 8x lower | Blood storage, transfusion |
| DEHT (DOTP) | 18x lower | General medical, cost-sensitive |
| TOTM | 100-350x lower | Drug infusion, chemotherapy |
A cardiac surgery study demonstrated the practical impact: TOTM migration in heart-lung machine tubing measured 350x lower than DEHP, with no cytotoxicity detected in TOTM metabolites. For extended blood contact during surgery, this difference matters.
Application-Specific Selection
Blood storage and transfusion: DINCH preserves red blood cell quality better than alternatives. Hemolysis rates at 35 days (0.297-0.342%) remain well below the 0.8% threshold, comparable to DEHP (0.204-0.240%). DINCH’s specific molecular design targets blood compatibility.
Drug infusion and chemotherapy: TOTM offers the lowest migration and best chemical resistance. For lipophilic drug formulations that could extract plasticizer, TOTM’s stability matters. I recommend TOTM for any extended infusion application.
Cost-sensitive general use: DOTP (also called DEHT) provides the easiest DEHP drop-in replacement. Processing parameters remain similar, and cost premiums typically stay below 10% of compound price.
The TOTM Contamination Paradox
TOTM has the best migration profile but carries a hidden compliance issue. The manufacturing process produces phthalic anhydride as a byproduct, resulting in inherent DEHP contamination up to 2,000 ppm. For applications requiring certified DEHP-free status, this contamination may disqualify TOTM despite its superior performance.
DOTP, when produced on dedicated equipment, achieves DEHP levels below 50 ppm. For strict “DEHP-free” certification requirements, DOTP may be the safer specification despite its higher migration rate.
This trade-off is something most suppliers do not discuss. Ask specifically about DEHP contamination levels when specifying TOTM for medical applications.
Key Takeaways
The regulatory landscape is converging around DEHP phase-out. Three deadlines define the transition window:
- May 2026: USP <665> takes effect
- December 2026: USP <88>/<87> updates
- July 2030: EU MDR full compliance; California restrictions effective
Four years is adequate for qualification and supply chain transition, but the window closes faster than most procurement cycles anticipate.
Plasticizer selection should match application risk. Blood contact favors DINCH. Extended drug infusion favors TOTM. Cost-sensitive general use favors DOTP. Requesting migration test data and DEHP contamination certificates from suppliers provides concrete evaluation criteria beyond marketing claims.
The shift away from DEHP is no longer a question of whether, only when and how smoothly. Starting qualification work now prevents supply disruptions when deadlines arrive.